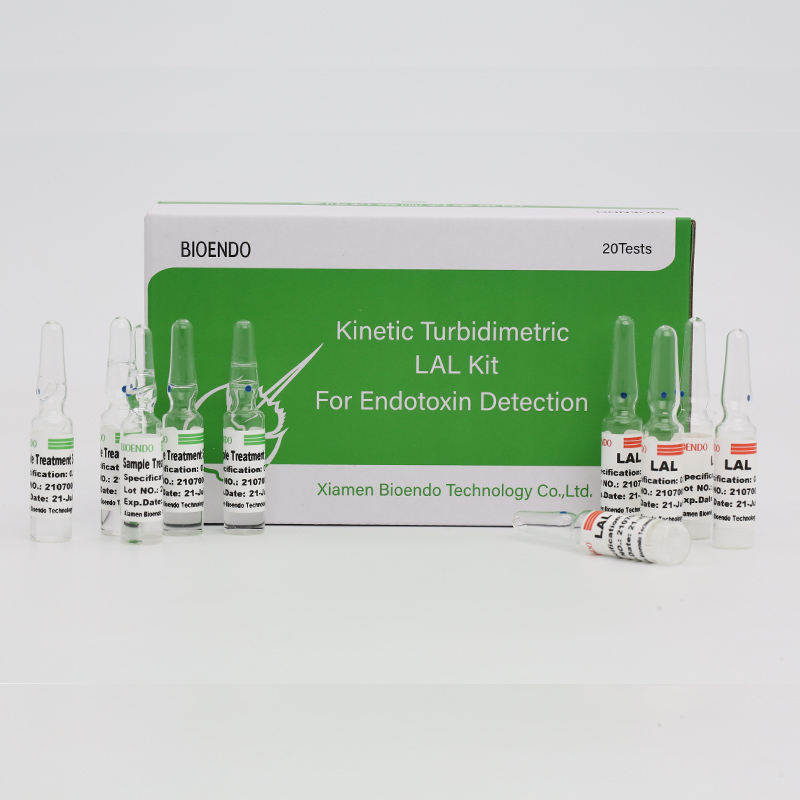
Endotoxin Assay Kit rau Tib Neeg Plasma
Endotoxin Assay Kitrau Human Plasma
1. Cov ntaub ntawv khoom
CFDA tshem tawmClinical Diagnostic Endotoxin Assay Kitquantifies endotoxin theem inhuman plasma.Endotoxin yog ib feem tseem ceeb ntawm phab ntsa ntawm tes ntawm Gram Negative kab mob thiab yog qhov tseem ceeb tshaj plaws microbial mediator ntawm sepsis.Kev nce qib ntawm endotoxin feem ntau tuaj yeem ua rau kub taub hau, hloov pauv hauv cov ntshav dawb suav thiab, qee zaum, mob plawv.Nws yog raws li qhov Cpathway hauv limulus Polyphemus (horseshoe crab blood) test.Nrog kinetic microplate nyeem ntawv thiab endotoxin assay software, Endotoxin assay cov khoom kuaj pom cov qib endotoxin hauv tib neeg cov ntshav hauv tsawg dua ib teev.Cov khoom siv los nrog cov ntshav plasma ua ntej kev kho mob reagent uas tshem tawm cov kev cuam tshuam hauv plasma thaum lub sijhawm kuaj endotoxin.
2. Khoom Parameter
Kev ntsuas ntau: 0.01-10 EU / ml
3. Khoom Feature thiab Daim Ntawv Thov
Los nrog cov kev daws teeb meem plasma pretreatment, tshem tawm cov teeb meem inhibition hauv tib neeg plasma.
Nco tseg:
Lyophilized Amebocyte Lysate (LAL) reagent tsim los ntawm Bioendo yog tsim los ntawm amebocyte lysate derived ntshav ntawm horseshoe crab.
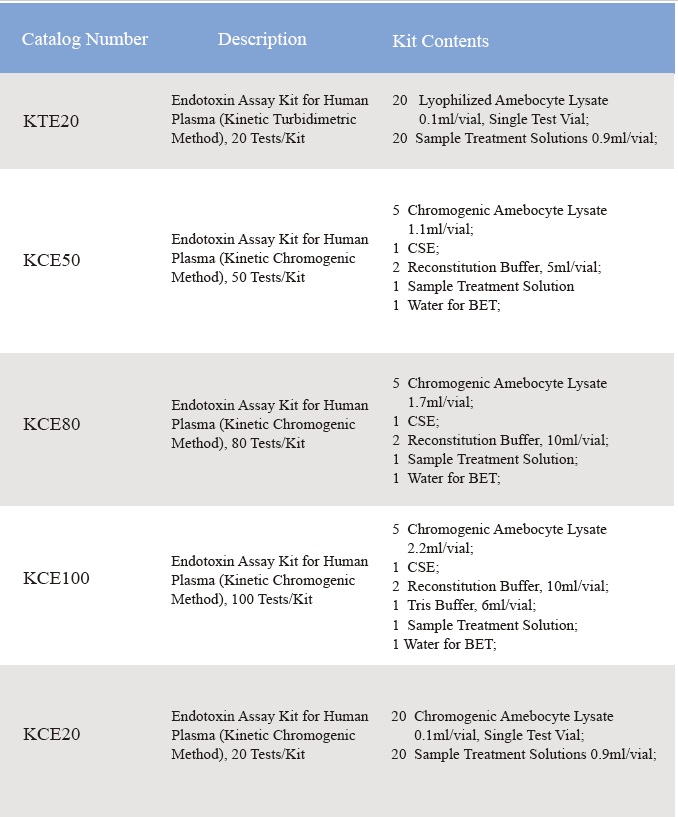
Lub rhiab heev ntawm Lyophilized Amebocyte Lysate thiab potency ntawm Control Standard Endotoxin raug soj ntsuam tawm tsam USP Reference Standard Endotoxin.Cov khoom siv Lyophilized Amebocyte Lysate reagent tuaj nrog cov khoom qhia, Daim Ntawv Pov Thawj Kev Ntsuam Xyuas, MSDS.